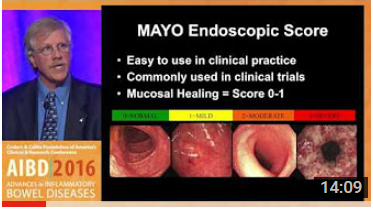
As noted in a previous blog post, endoscopic remission in the treatment of Ulcerative Colitis (UC) increasingly is being relied upon as an objective measure to assess a drug’s efficacy.
In the Company’s ongoing Phase 2 Proof-of-Concept (PoC) trial of Brilacidin in Ulcerative Proctitis (UP) / Ulcerative Proctosigmoiditis (UPS), two types of Inflammatory Bowel Disease (IBD), a total of 17 patients across three sequentially recruited cohorts will have enrolled to receive Brilacidin once daily administered per rectum as a retention enema for 42 days (6 weeks) of treatment. Cohort A (6 patients) and Cohort B (6 patients) have already completed treatment, receiving 50 milligrams (mg) and 100 mg of Brilacidin, respectively. Cohort C (5 patients) currently is in progress, with patients receiving 200 mg once daily.
For all patients, endoscopic evaluation of the rectum and mucosa up to 40 cm from the anal verge will be performed at screening and at the end of treatment/Day 42 (± 3 days), with the scoring done by trial investigators. Following study completion, similar endoscopic evaluation will be conducted by a panel of independent practicing gastroenterologists (identified as key opinion leaders) drawn from leading teaching hospitals. Faecal and other biological markers also will be reviewed to establish Brilacidin's therapeutic effect.
Based on interim results in the Brilacidin-UP/UPS study, which is now nearing completion, the Company has seen clinical remission in half of all patients (6 of 12) treated so far, including noticeable reductions in ulceration and bleeding in most patients (7 of 11), based on endoscopic review.
Dr. Francis A. Farraye, one of the Company's scientific advisors, is an expert in IBD (list of publications) and recently presented at the 2016 Crohn’s & Colitis Foundation of America’s Clinical Research & Conference. His talks are linked to below, the first of which — “How Can We Best Determine Endoscopic Severity in Ulcerative Colitis and Crohn’s Disease” — discusses endoscopy in IBD. The second talk addresses health maintenance in IBD by preventing of infectious diseases due to immunosuppression.
Topline results for the Brilacidin UP/UPS trial will be presented at the 2017 Drug Discovery and Therapy World Congress to be held July 10 – 13, 2017 in Boston.